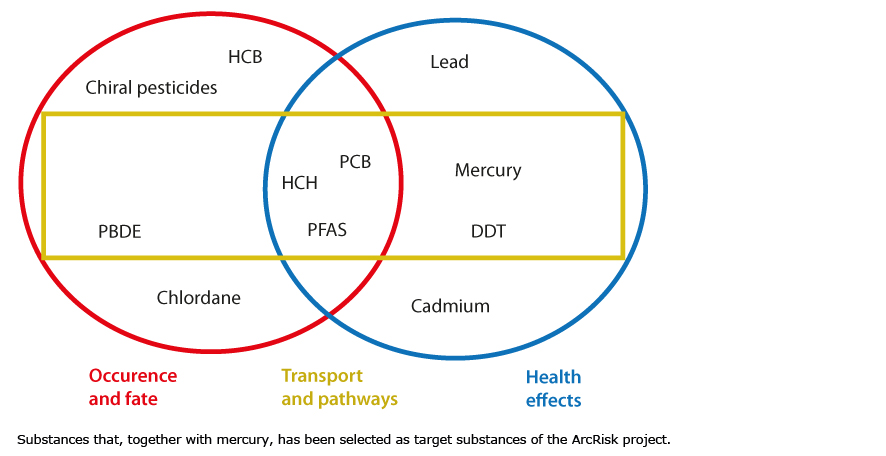
Target Substances
Several POPs and heavy metals were studied in the ArcRisk project. A core set of substances were investigated more thoroughly. They are identified at the centre in the figure below, in the area encompassed by all parts of the project. All substances investigated are of concern with regards to Arctic food webs and human food.
This set of chemicals can be classified into three broad groups: the “legacy” persistent organic pollutants (POPs), “emerging” substances and heavy metals. The “legacy” POPs are members of the original group of identified in the Stockholm Convention. These include PCBs and organochlorine pesticides, such as chlordanes and DDT. In 2009, nine additional substances were added to the Stockholm Convention, including HCH, PBDEs and PFOS. Cadmium, lead and mercury are examples of heavy metals that have been studied within ArcRisk.
Cadmium
Chlordanes
Chiral pesticides
Dichloro-diphenyl-trichloroethane (DDT) and its metabolites
Hexachlorobenzene (HCB)
Hexachlorocyclohexane (HCH)
Lead
Mercury
Perfluorinated Alkylated Substances (PFAS)
Polybrominated diphenyl eters (PBDE)
Polychlorinated biphenyls (PCB)
Chlordanes
The technical chlordane mixture consisted mainly of trans- and cis-chlordane, followed by trans-nonachlor and heptachlor. Chlordanes have been used as crop pesticide and as termiticide in buildings. Indoor air has therefore been important for human exposure (Stockholm convention). Chlordanes are transported to remote areas where they may bioaccumulate in the food web, and hence, food is the major exposure route for people outside areas where chlordane have been used indoors (Deutch et al., 2004; AMAP, 2009). Chlordanes affect the reproduction and immune systems (AMAP, 2009). Trans-, cis- and oxychlordane and some of the minor components of technical chlordane are chiral.
 Trans-chlordane is one of the chlordanes studied in the ArcRisk project.
Trans-chlordane is one of the chlordanes studied in the ArcRisk project.
Chiral pesticides
The pesticides α-hexachlorocyclohexane (α-HCH), trans-chlordane, cis-chlordane and the chlordane transformation product oxychlordane are chiral. Chirality means that a molecule has two (or more) non-superimposable enantiomers. Both enantiomers have the same physical-chemical properties, but differ in the 3D-structure –like your right and left hand! The enantiomers can rotate plan-polarised light in different directions and get a “+” or a “-“ symbol in front of the compound name to differ between the enantiomers.
The enantiomers of a substance will be affected similarly by physical transport processes within the environment because enantiomers have identical physical/chemical properties. However, within biological systems, enantiomers will behave like separate substances because they interacting with other enantiomers in a homochiral environment (Kallenborn and Hühnerfuss, 2001). Most technical mixtures of chiral pesticides were sold in a racemic mixture. Eventually, due to biochemical processes, the enantiomeric fraction (EF; eq. 1) will change from a racemic, 50:50 relationship between the (+)-enantiomer and the (-)-enantiomer to an enrichment/degradation of one of the enantiomers. The EFs of pesticides can therefore be used as a tool to distinguish between primary, fresh sources and secondary, weathered sources of contaminant exposure in the environment (Bidleman et al., 1998; Bidleman and Falconer 1999; Wong 2006).
Chiral contaminants in low trophic level organisms like marine zooplankton are considered to reflect the enantiomeric signature of the surrounding environment, i.e. the water column (Hoekstra et al., 2003). Hence, non-racemic EFs of chiral pesticides in zooplankton would be related to the surrounding environment and changes therein, such as ice cover/break up, ocean currents, revolatilisation from old sources and atmospheric exchange (Carlsson et al, manuscript).
Dichloro-diphenyl-trichloroethane (DDT)
DDT is a pesticide extensively used from its introduction in the 1940’s until its ban in most countries in the 1970’s. DDT is still in use in some countries to control mosquitos carrying malaria.
The commercial mixture of DDT contain several isomers, and the main constituent is the p,p’-DDT-isomer. The two main metabolites of DDT are dichlorodiphenyldichloroethylene (DDE) and dichlorodiphenyldichloroethane
(DDD), which are also, although only to a small extent, included in the commercial mixture (WHO 2009). DDTs are persistent substances and they remain in the nature although the extensive use ended rather a long time ago. The distribution of DDT contamination is world-wide.
DDT causes egg-shell thinning in birds’ eggs, which gave rise to major concerns in the 1970’s when eagle populations were strongly affected. Effects from short term exposure of humans are limited but if long-term exposure occurs it can result in chronic effects. DDT is a POP under the Stockholm Convention.
Hexachlorobenzene (HCB)
HCB has previously been used as a fungicide, but is today released as a by-product of the (pesticide and chemical) industry and it may be formed unintentionally during combustion. It is volatile and undergoes long-range transport to the Arctic (AMAP, 2002). Today, atmospheric levels of HCB seem to decrease in the Arctic, but not at the Zeppelin station (78o55’ N, 11o56’ E; Ny-Ålesund, Svalbard). This is probably due to evaporation of HCB from ice free waters to the atmosphere (Hung et al., 2010; Ma et al., 2011). HCB can affect reproduction in both human and other animals (Stockholm Convention, 2013).
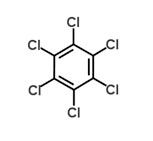
Hexachlorocyclohexane (HCH)
Technical hexachlorocyclohexane (HCH) was one of the most widely used pesticides during the 20th century. There are several forms (isomers) of HCH and technical HCH is an isomeric mixture of mainly five forms of HCH.
γ-HCH, or lindane which is the common name, is a broad spectrum insecticide which is still produced but to a much lesser extent nowadays compared to a few years ago. α-HCH and β-HCH are unintentionally formed by-products in lindane production. Although production and use were phased out during the 1990s, HCHs are still ubiquitous in the environment and re-emissions still occurs from stockpiles and contaminated sites. Emissions of α - and β -HCH have decreased since the 1980s (Li et al., 2000). The HCHs are persistent and are subject to long-range transport, bioaccumulation and are toxic to both humans and wildlife (Stockholm Convention). α-HCH, β-HCH and γ-HCH are POPs under the Stockholm convention.
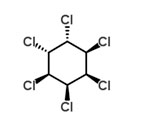
Mercury
Mercury is one of the most toxic metals and occurs throughout the environment as a consequence of natural sources and human activity. Coal incineration is the main anthropogenic sources of mercury. Artisanal/gold-mining are also a large source (Pacyna et al., 2010).
Mercury can be divided into three categories: the metallic form, also called the elemental form; the divalent inorganic forms; and organic mercury compounds, all having different toxicokinetic properties. Some microorganisms and natural processes can change mercury in the environment from one form to another. The most common organic mercury compounds that microorganisms and natural processes generate from other forms are monomethylmercury compounds. These forms of mercury accumulate in the food chain and can build up in certain edible freshwater and marine fish and marine mammals to levels that are many times higher than the levels in the surrounding water. Inorganic Hg compounds accumulate up the food chain to a much smaller degree.
Perfluorinated alkylated substances (PFAS)
The PFAS group includes perfluorinated sulfonates and carboxylic acids as well as fluorotelomer alcohols. Only perfluorooctane sulfonic acid, its salts and perfluorooctane sulfonyl fluoride (PFOS-F) are listed within the Stockholm Convention, but usage and production are still allowed for several purposes.
Due to their surface active properties, PFAS compounds have been used extensively in e.g. firefighting foam, GoreTex®, Teflon®, and as emulsifiers. Due to their surface properties, PFAS can cause effects on intracellular organelles, the liver, immune and hormone systems (Donaldson et al., 2010, Donaldson et al., 2012). PFAS bind to proteins instead of lipid rich tissues as other POPs do (AMAP 2009, Donaldson et al., 2010).
Polybrominated biphenyl ethers
Polybrominated diphenyl ethers (PBDEs) have been used as flame retardants in various materials, such as electric equipment, textiles and plastics. The technical mixtures penta- and octaBDE have been banned since 2004 in the EU and Norway, and the production in USA was voluntarily ceased in 2005 (BSEF, 2013). PBDEs were listed under the Stockholm convention in 2009. PBDEs, and especially hydroxylated PBDEs have been reported to interfere with oestrogen and thyroid receptors (Darnerud et al., 2001; Meerts, 2001; de Wit, 2002).
Polychlorinated biphenyls (PCBs)
PCBs are a group of 209 substances, which have the same basic structure but different degree and of chlorination and orientation of the chlorines. 13 of the PCBs exhibit dioxin-like activity (►), due to their planar structure. PCBs are toxic to fish and may cause reproductive failure and suppression of the immune system in seals, minks and other animals. Negative effects in humans are damage to the immune system, liver, skin, reproductive system, gastrointestinal tract and thyroid gland (Stockholm convention).
PCBs were produced extensively during the 1950-70s. They were used as coolants and insulating fluids in electrical equipment, in paint and as additives and sealants in building materials. After the 1970s, production and use of PCBs were restricted in many countries. However, the decline in emissions lags strongly behind the rate of phase-out, because products and materials continue to release PCBs to the atmosphere throughout their lifetime. The major parts of emissions today come from secondary sources, such as remobilisation from PCB reservoirs in sediment, water, soil, snow and ice (Stockholm convention).
Despite the ban, PCBs are widespread in the environment as they are persistent and bioaccumulative. PCBs are listed at the Stockholm convention. PCBs are one of the best-understood groups of POPs in terms of physical-chemical properties, emissions, and observed concentrations in the atmosphere. Therefore, they are a valuable case study for evaluating the performance of models and as a benchmark for other substances in the climate change scenarios.
(►) Dioxin-like activity: Dioxins are toxic substances causing a number of negative effects in humans and animals, such as reproductive effects, developmental effects, damages to the immune system, hormonal effects as well as cancer. These effect are mediated via a receptor called the aH-receptor and thus other substances, similar in structure to the dioxins, may cause similar effects. (see e.g. WHO 2010 and Parkinson 2001)