Abiotic occurence and processes
Several field campaigns have been carried out as a part of the ArcRisk project. Data from these campaigns have been used for validation of models and to investigate transport processes, especially the link between abiotic and biotic environment. Transfer of POPs from abiotic matrices into biota is an important process, but there are few studies available. This page gives some of the ArcRisk results, and provides links to databases, not only from reviews conducted in the ArcRisk project, but also links to databases where large data sets and information are stored.
Databases of contaminants
Several field campaigns have been conducted in Arctic areas during the last decade. Within the ArcRisk project, an overview over POP levels has been established in 2010. It contains abiotic compartments such as air, snow, ice and water, as well as flux of contaminants within snowpacks and ice caps. In addition, low trophic level animals (plankton) have been included. Hence, this database provides an overview over recent data available. More studies are available, especially from the years after this overview was constructed.
An ISI ‘Web of Science’ and PubMed Literature survey (12.01.2012) revealed that about 1200 publications are available in peer-reviewed international journals on POPs in Arctic environments (since 1979). The ArcRisk project has compiled an overview of recent (1999-2011) and relevant reviews on fate and distribution of POPs and mercury in Arctic and Antarctic biota. The list can be downloaded here lænk till listan “relevant reviews on fate and distribution of POPs and mercury in Arctic and Antarctic biota” in D26. Information about analytical methods and sampling strategies are available in several peer reviewed articles.
Environment Canada provides regular reports on POPs levels in the North American Arctic as a part of the Northern Contaminants Program (NCP). These data are available on the internet - Canadian Arctic Contaminants Assessment Report. The written reports can be ordered directly from the NCP secretariat.
Environmental fate of organic contaminants
POPs can be transported from the primary source to remote areas. How far, when and how they will be transported is determined by their physical-chemical properties.
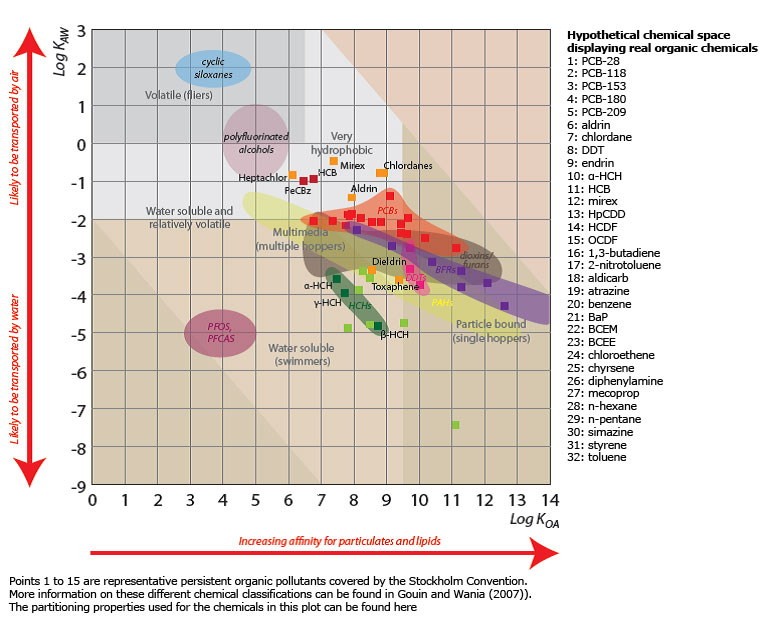
Changed temperatures and precipitation patterns can affect the abiotic transport processes as well as and their uptake in biota. Snow and ice melt brings “stored” POPs to the receiving rivers and oceans. Invading species might bring pollutants into the ecosystem, species composition might change in the ecosystems and the primary production can be affected as well. These are all examples of factors and processes that the ArcRisk project aimed to elucidate.
Fate processes in snowpack
In areas of high latitude (and altitude) snow is a significant source of atmospherically derived contaminants to catchment areas as well as marine waters (Blais et al., 2001; Daly and Wania, 2004; Bergknut et al., 2010; Pućko et al., 2011). However, the release of contaminants from the snowpack and their geochemistry following periods of thaw are not well understood, particularly for chemicals that are generally more polar, ionizable and/or largely non-volatile compared to semi-volatile “legacy” POPs. To date, there is a lack of measurement data for these chemicals in the remote snowpack.
Because snow accumulation on terrestrial surfaces or sea ice is controlled by several snowpack temperature- and wind-associated processes (e.g. wind pumping) that influence its structure and moisture content (Halsall, 2004; Herbert et al., 2006), chemical phase partitioning between vapour, particles, water and ice is crucial to understand the fate of contaminants in the snowpack. When melting commences with a seasonal increase in air temperature, chemical repartitioning can occur within the snowpack, which in turn will influence their elution order and release with meltwater (Halsall, 2004; Durnford and Dastoor, 2011; Meyer and Wania, 2010).
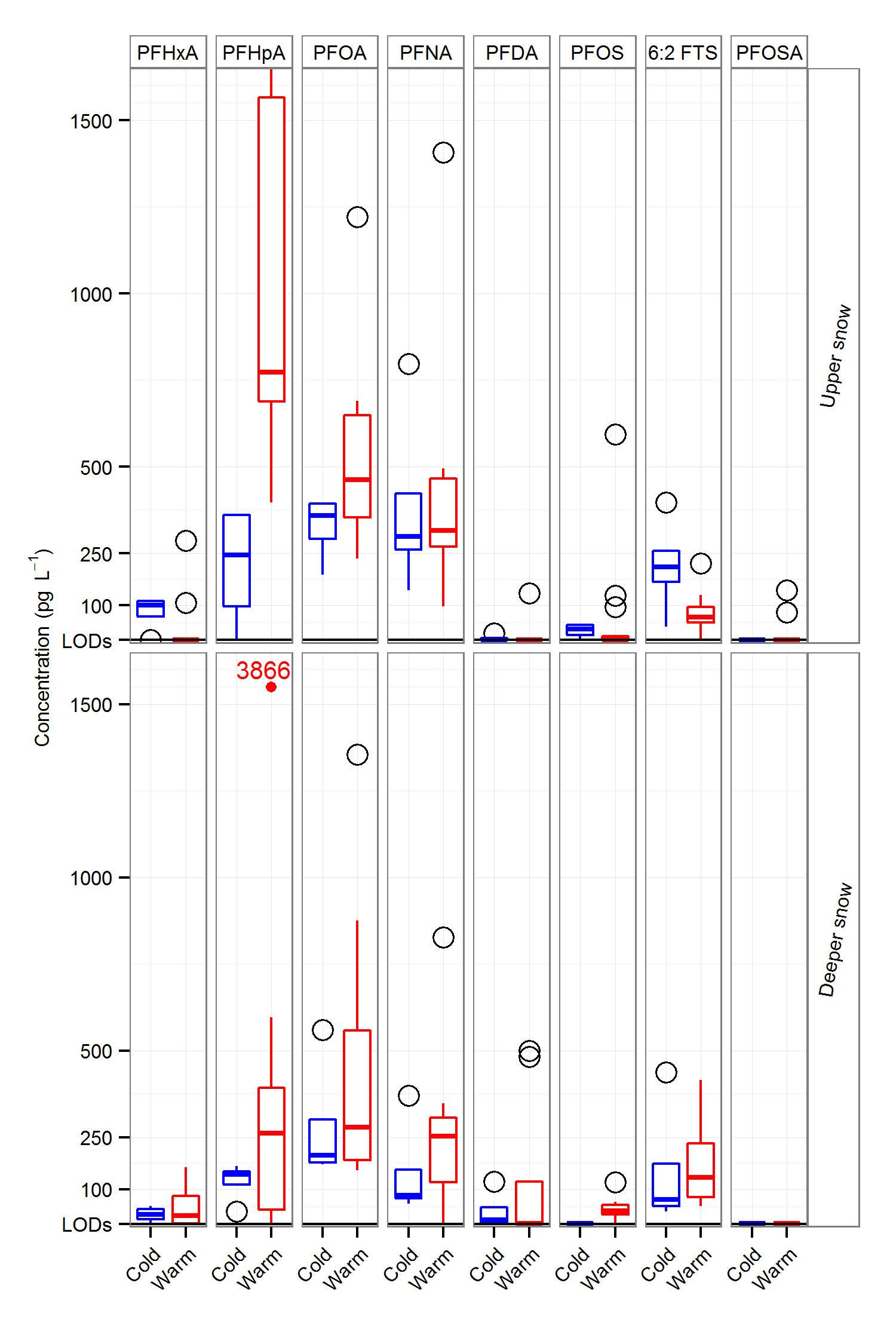
The geochemical behaviour of substances such as mercury and PFAS in the ageing snowpack is controlled by the physical properties of the snow and chemical processes (e.g., reduction and oxidation of Hg, and ice-based chemical partitioning coefficients for POPs), as well as particle type and amount. These interactions “decides” the fate of chemicals within the snowpack and control their elution during melt, either in a “first flush” (e.g., reactive gaseous mercury (RGM) and some shorter chain PFAs, such as perfluorobutane sulfonate (PFBS)), or later for particle-associated chemicals e.g. PHg). Despite the fact that PFAS are more water soluble than hydrophobic POPs, their various chain lengths and surfactant properties ensure a variable elution order during the onset of melt, although recent fieldwork conducted as part of the ArcRisk project shows that over time these chemicals will accumulate and undergo enrichment in snow layers prior to final melt.
Organic contaminants in the snowpack
An important process that often accounts for the loss of hydrophobic, semi-volatile chemicals following compaction of fresh snow layers is snow-air exchange. Revolatilisation of ice-sorbed contaminants from fresh snow has been observed in several Arctic studies, and is perhaps one of the largest loss processes of semi-volatile POPs from the ageing snowpack prior to the onset of melt (Herbert et al., 2005; Pućko et al., 2010, 2011). For non-volatile or ionizable chemicals such as the perfluoroalkyl acids, revolatilization can be considered to be negligible following initial snow compaction, ensuring that the mass deposited in successive snowfall events will be retained in the snowpack until melt processes commence.
PFAS
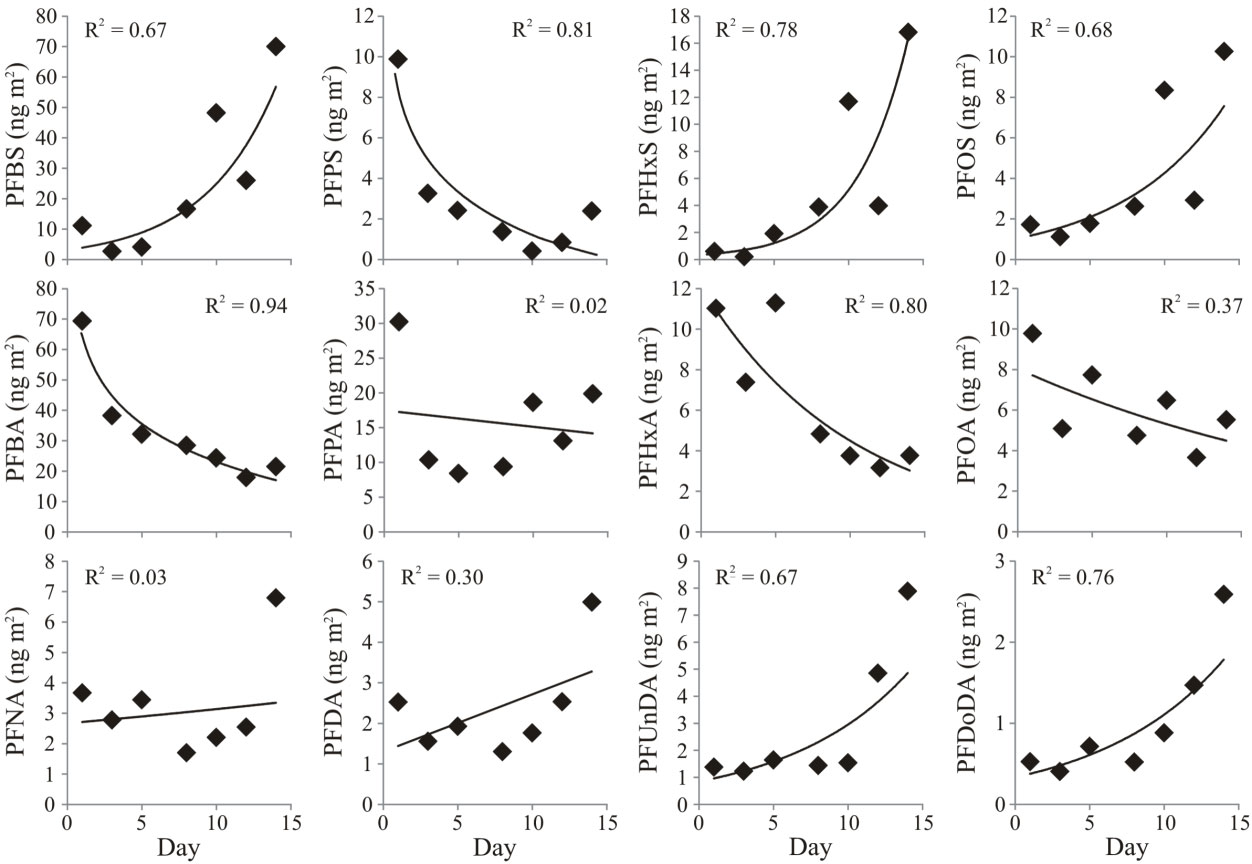
PFAS are deposited from the atmosphere to the seasonal snowpack via snowfall, especially during the late winter. Once in the snowpack, PFAS can migrate between different layers of snow, depending on the liquid water content in these snow layers. Their “elution order” from the melting snowpack depends on their physical-chemical properties. The short-chained PFCAs (4-9 carbon atoms in the molecule; C4-9) are likely to be released first, and reach surface waters via the meltwater runoff. Those PFCAs with longer carbon chains (C10-12) and the PFSAs are more likely to be retained within the snowpack and be released directly into the ground at the base of the snowpack, see picture below.
PCBs, PBDEs and pesticides
ΣPCBs and ΣPBDEs loads in a melting seasonal snowpack showed a gradual decrease over a two week period with a significant inverse correlation with a nearby stream flow. On the other hand, HCHs were not correlated with stream flow and instead showed a marked rapid decline in their snowpack load during the first few days of melt. Similarly, p,p’-DDT, p,p’-DDE and other predominantly particle-bound chemicals did not reveal any trend with either snowpack depth or stream flow. Low particle-bound fractions (<0.4) for several of the higher brominated PBDEs were observed in the late-season snowpack (i.e. BDE-153, -138 and -183), but not for the migh-chlorinated PCBs. This suggests novel formation in the snowpack (possibly due to photochemical debromination of nona-BDEs and deca-BDE) rather than input through particle-bound deposition from the atmosphere.
The input to receiving waters during snowmelt will follow a certain order, due to slightly different properties among the POPs, rather than delivered as a single ‘pulse’ with all contaminants delivered at the same time. However, a rapid thaw of the snowpack during anomalous winter warming events may alter this behaviour.
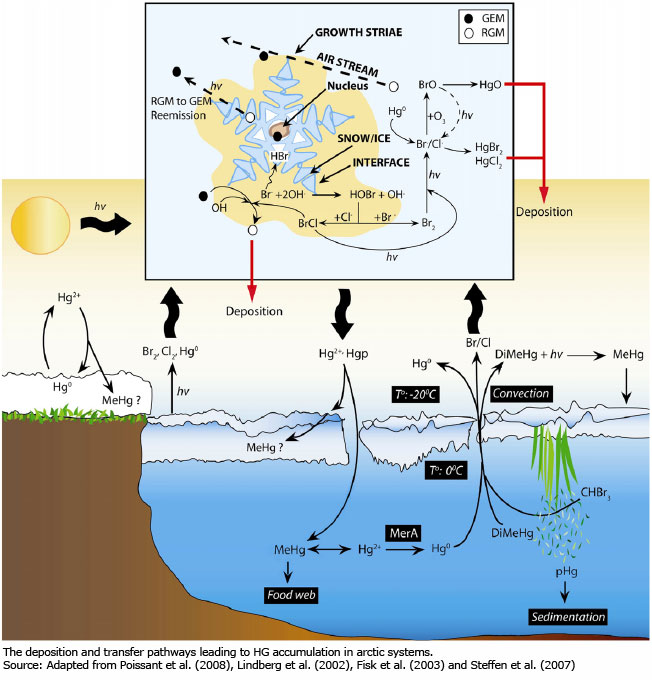
Mercury
Gaseous elemental mercury (GEM or Hg0) is transported via long-range atmospheric transport to the Arctic. Sunlight, ozone, and bromine-mediated chemical reactions convert mercury into oxidized RGM, which can be associated with particles (PHg), and all mercury species are deposited (Lindberg et al., 2002; AMAP, 2011; Durnford and Dastoor, 2011). Following polar sunrise these reactions cause atmospheric mercury depletion events (AMDEs) which largely account for the majority of Hg deposition and accumulation in fresh snow (AMAP, 2011, Douglas et al., 2005; Durnford and Dastoor, 2011, Lindberg et al., 2002, Wang et al., 2011). Furthermore, frost flowers that develop on recently produced sea ice concentrate mercury up to nine times compared to coastal areas and contribute to the complex chemistry of AMDEs in the weeks following polar sunrise and before snow melt (Douglas et al., 2005, Lindberg et al., 2002; AMAP, 2011a). Approximately 80 percent of Hg deposited in snow can be transformed to GEM and revolatilize back to the atmosphere, with the remaining fraction available to meltwater for microbial conversion to alkylated forms, e.g., methylmercury (MeHg) (AMAP, 2011a). However, in the pelagic marine environment, during the melt period in early summer, the remaining Hg will leach from the snowpack and contribute to Hg concentrations observed in the beneath-ice seawater (Chaulk et al., 2011; Wang et al., 2011).